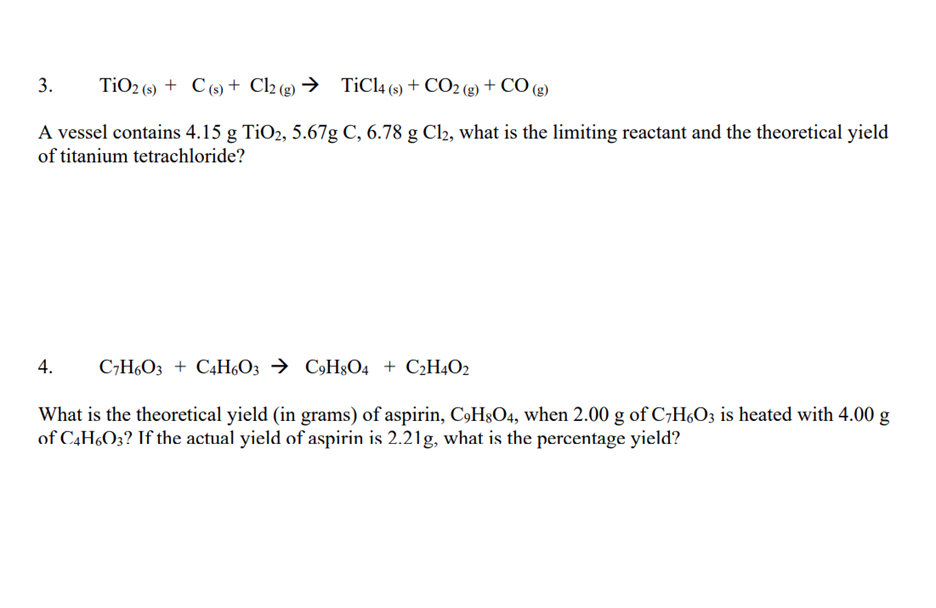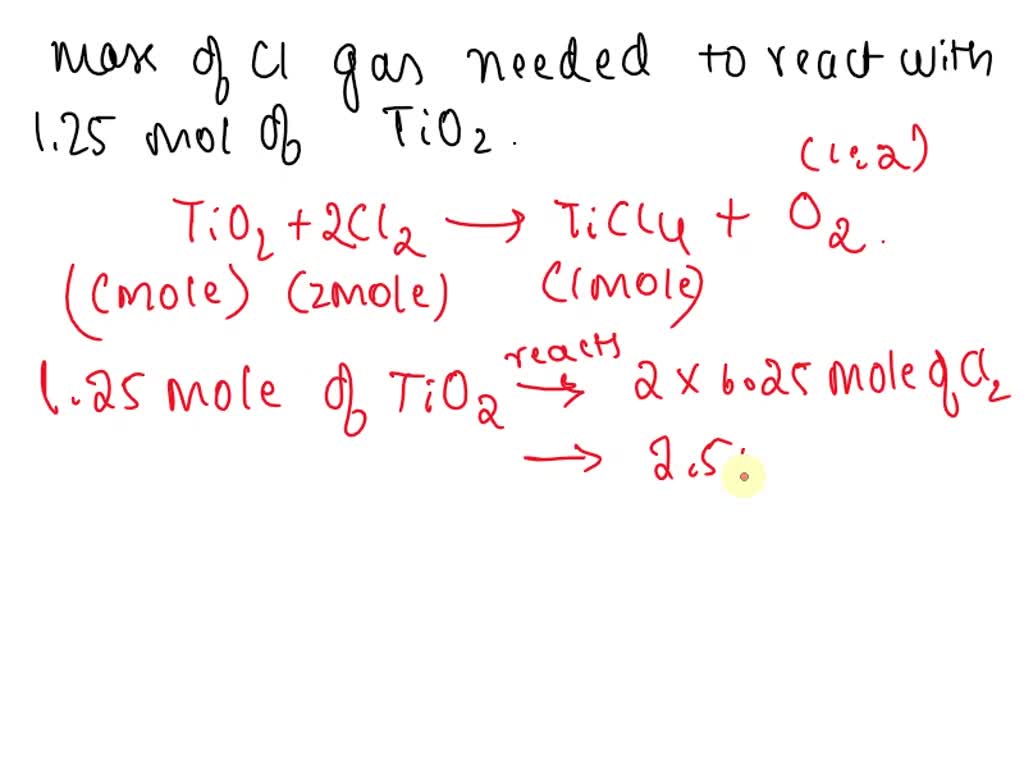Cl2 Production by Photocatalytic Oxidation of HCl over TiO2 - Rath - 2019 - ChemSusChem - Wiley Online Library
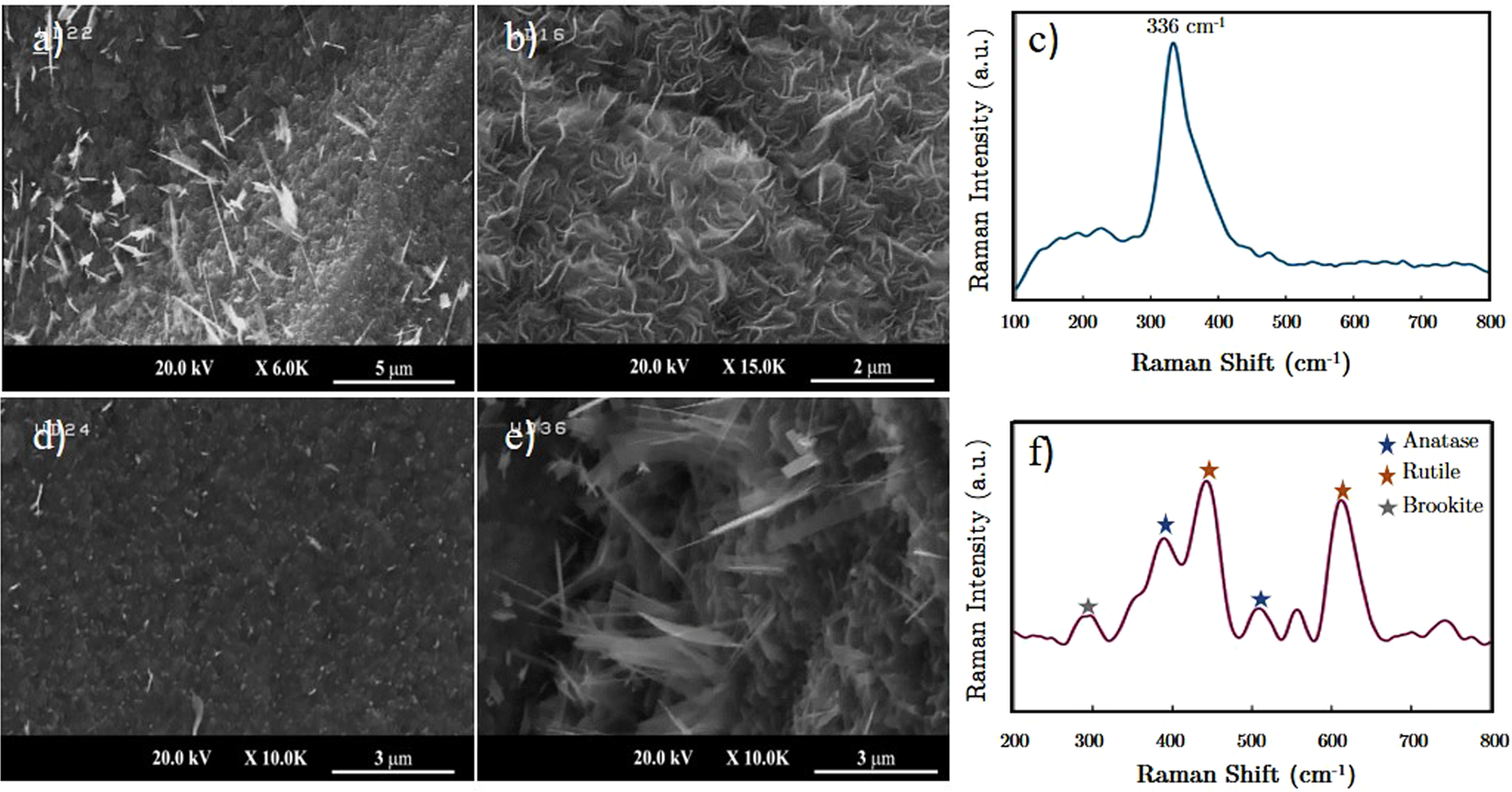
Evolution of large area TiS2-TiO2 heterostructures and S-doped TiO2 nano-sheets on titanium foils | Scientific Reports

Catalysts | Free Full-Text | Heterogeneous Photocatalytic Chlorination of Methylene Blue Using a Newly Synthesized TiO2-SiO2 Photocatalyst

High-resolution TEM images of Pt/TiO2 (a), Au/TiO2 (b), Pd/TiO2 (c) and... | Download Scientific Diagram
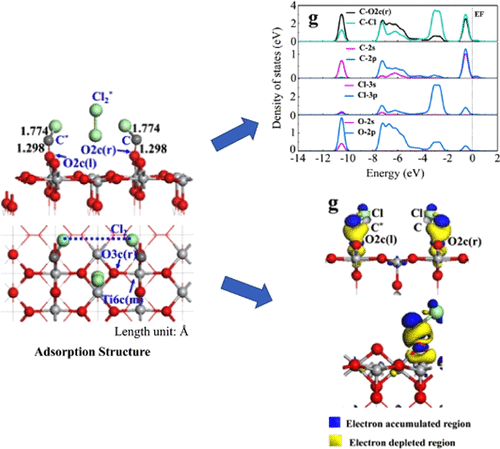
Prediction of Structural and Electronic Properties of C and Cl2 Adsorbed on the Rutile TiO2 (110) Surface,ACS Omega - X-MOL

Dependence of Cl 2 concentration as a function of (a) amount of coated... | Download Scientific Diagram

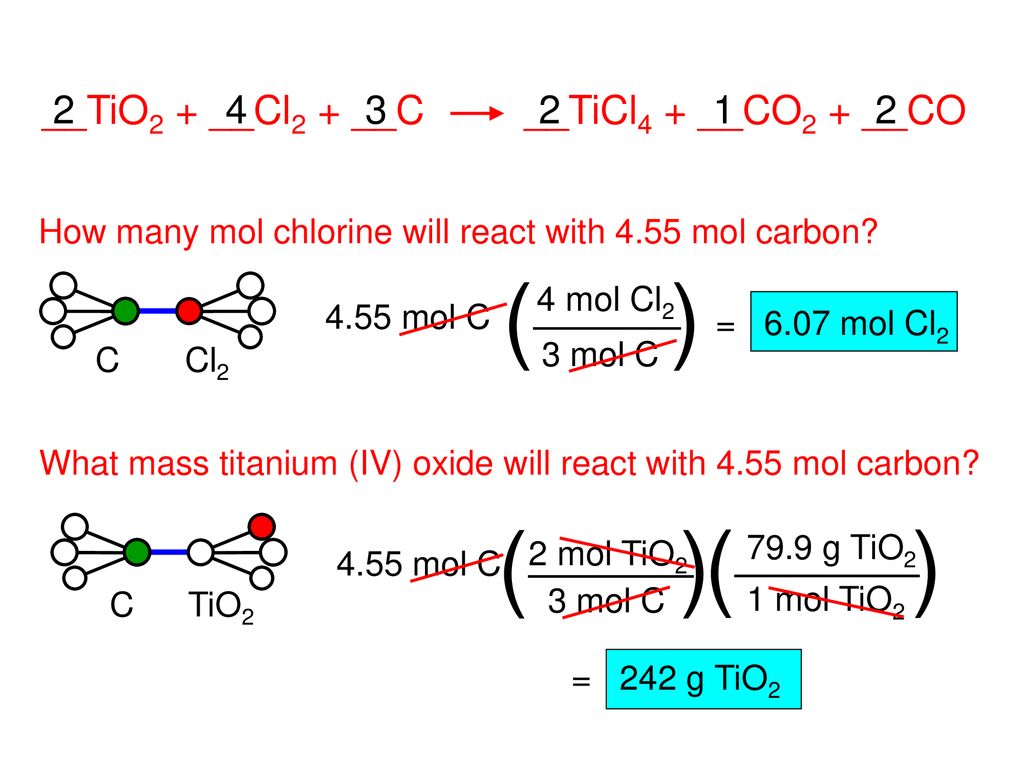
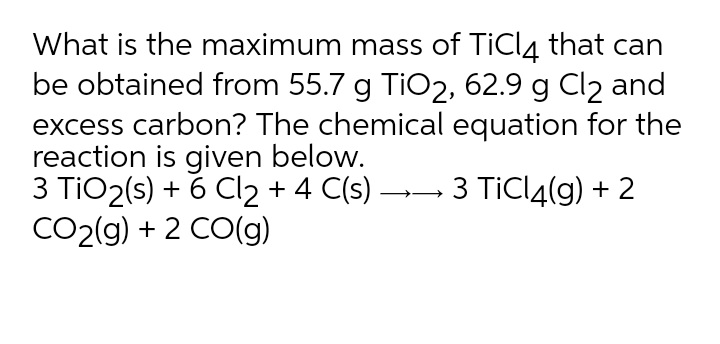
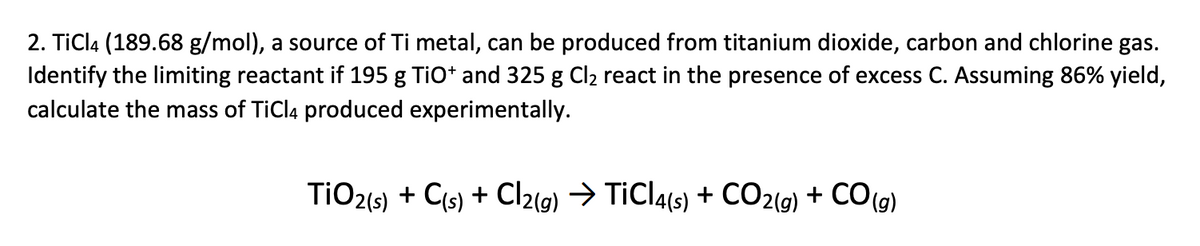
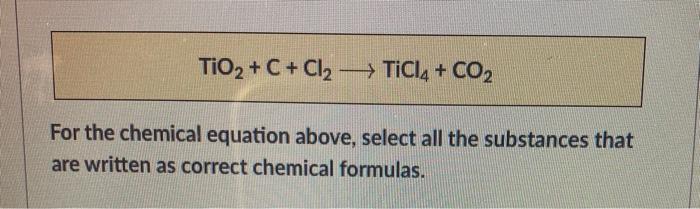
SOLVED: Challenge Titanium is a transition metal used in many alloys because it is extremely strong and lightweight. Titanium tetrachloride (TiCl4) is extracted from titanium oxide (TiO2) using chlorine and coke (carbon).
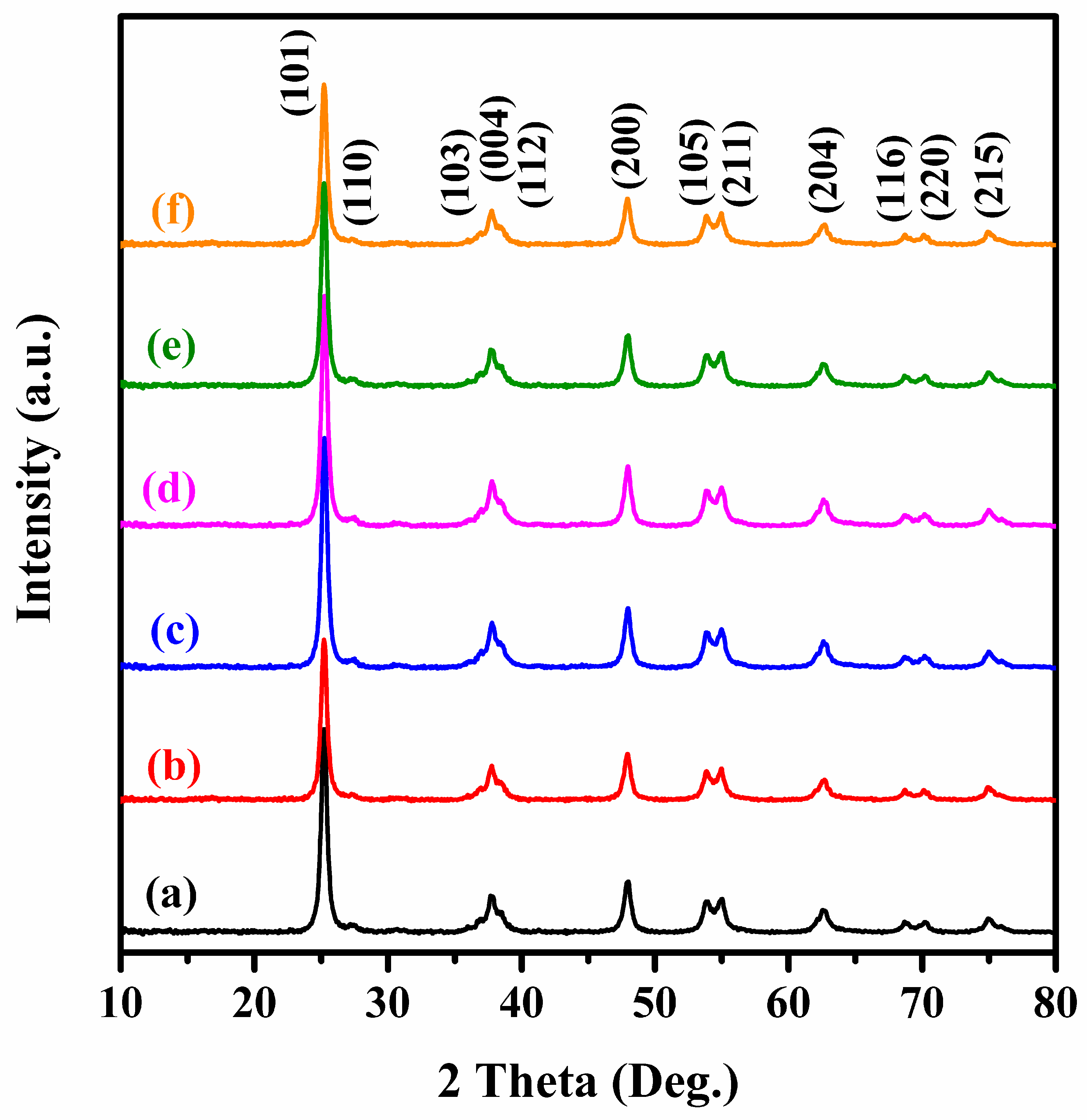
Catalysts | Free Full-Text | AuPd/3DOM TiO2 Catalysts: Good Activity and Stability for the Oxidation of Trichloroethylene